Soft biorobotic heart, computational fluid models, aid in evaluation of surgical techniques for Tetralogy of Fallot, a congenital heart defect
Soft robotic biohybrid heart and computational fluid models support valve assessment for Tetralogy of Fallot, a heart defect in newborns
Tetralogy of Fallot (ToF) is a critical congenital heart defect. Babies born with ToF have four cardiac anomalies: an enlarged right ventricle, a hole in the wall separating the right from the left chamber, narrowing of the pulmonary artery (the vessel that carries blood to the lungs), and the aorta receives blood supply from both ventricles. The condition, which impacts roughly 2,000 infants each year, makes it difficult for the baby’s heart to send enough oxygen to their entire body and requires corrective surgery in infancy.
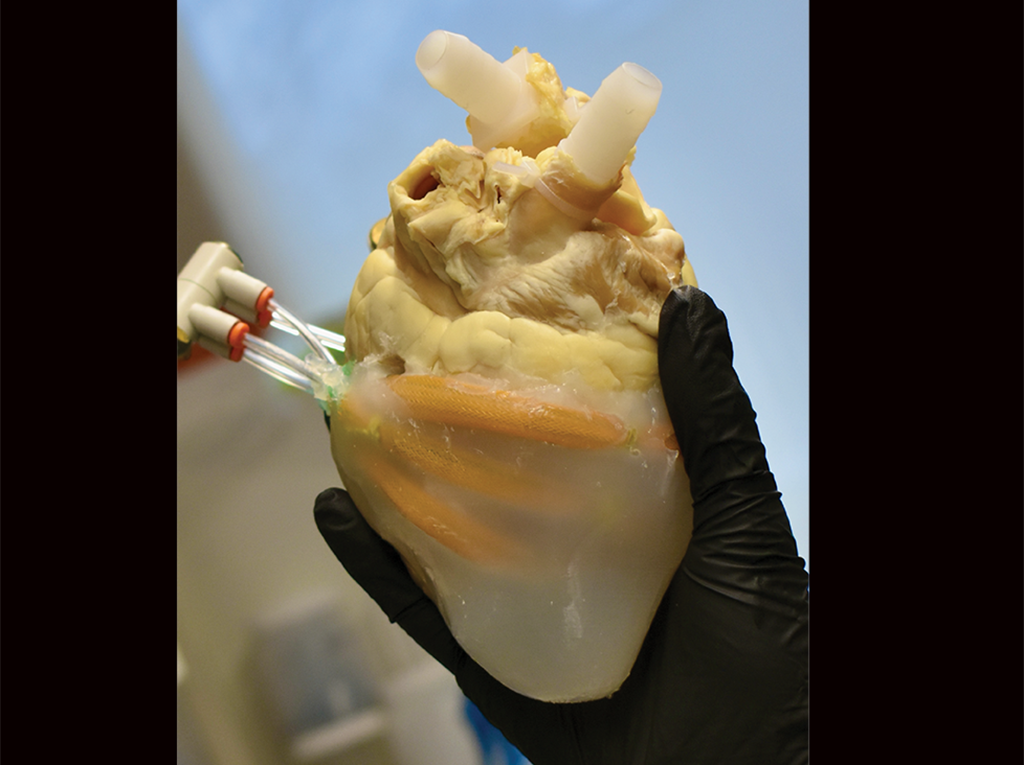 A soft robotic biohybrid heart (shown) paired with computational fluid models supports valve assessment for Tetralogy of Fallot, a heart defect in newborns. (Image: Courtesy of the researchers).
A soft robotic biohybrid heart (shown) paired with computational fluid models supports valve assessment for Tetralogy of Fallot, a heart defect in newborns. (Image: Courtesy of the researchers).Surgical interventions, while necessary, can cause issues, including pulmonary valve insufficiency, progressive right ventricle failure, or valve failure. A new study published in the journal Science Translational Medicine aims to lessen these issues by identifying optimal surgical approaches.
“We were interested in investigating different approaches to surgically repair a part of the heart called the right ventricular outflow tract (RVOT), the conduit where the blood flows from the right side of the heart to the lungs for oxygenation,” says Ellen Roche, Latham Family Career Development Professor and associate professor of mechanical engineering.
The researchers developed a model of an impaired right ventricular outflow tract function in a soft biorobotic heart and paired with a computational hemodynamic (blood flow) model of the RVOT to allow comparison surgical techniques and identify would be most beneficial based on a patient’s blood flow patterns. They also investigated a monocusp valve made from an entirely biological cell-assembled extracellular matrix (CAM) designed to tackle the multifaceted issue of monocusp failure.
The research was conducted in collaboration with a group in Bordeaux, France, with funding through the MISTI-France award. Fabien Kawecki, now an independent researcher, came from Bordeaux to visit Roche’s lab, and Manisha Singh, a postdoc in Roche’s group who spent time working in Bordeaux, are the paper’s lead authors.
“This was a great collaborative study and we hope that it can help with investigating patient specific repair strategies for patients with ToF or other congenital heart defects,” says Roche.


